How GENV-HEM (AAV8.FVa) works
High Affinity Delivery
We utilize AAV vectors with high target tissue affinity to deliver optimized transgenes that hold the potential to serve as medicines to treat inherited diseases.
Scientific and Regulatory Strategies
Our Scientific Team has over 28 years of experience with AAV gene therapy, from vector design to transgene optimization. Our Regulatory Team has a track record of FDA approvals and has worked on over 100 programs ranging from IND to NDA and BLA. Together our Scientific and Regulatory Teams work collaboratively with our investigators conducting contract and sponsored research to assess the efficacy and safety of our gene therapy product candidates.
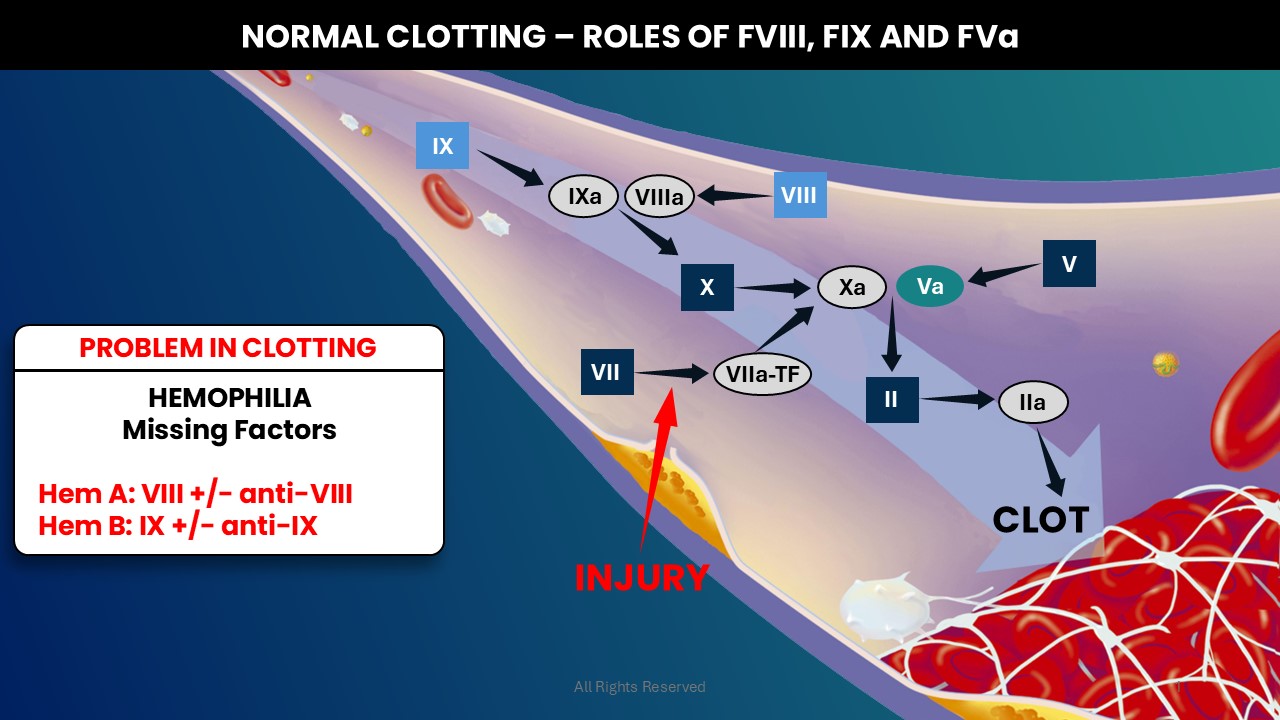
In healthy individuals, an injury triggers the coagulation cascade. Coagulation Factors VIII and IX are pivotal proteins for the generation of activated Factor X (Xa), which in complex with activated Factor V (Va), leads to thrombin (IIa) production culminating in the generation of a clot. In hemophilia, deficiencies of Factor VIII or IX, or in the presence of their neutralizing antibodies (anti-FVIII and anti-FIX, termed inhibitors), clot formation is significantly impaired.
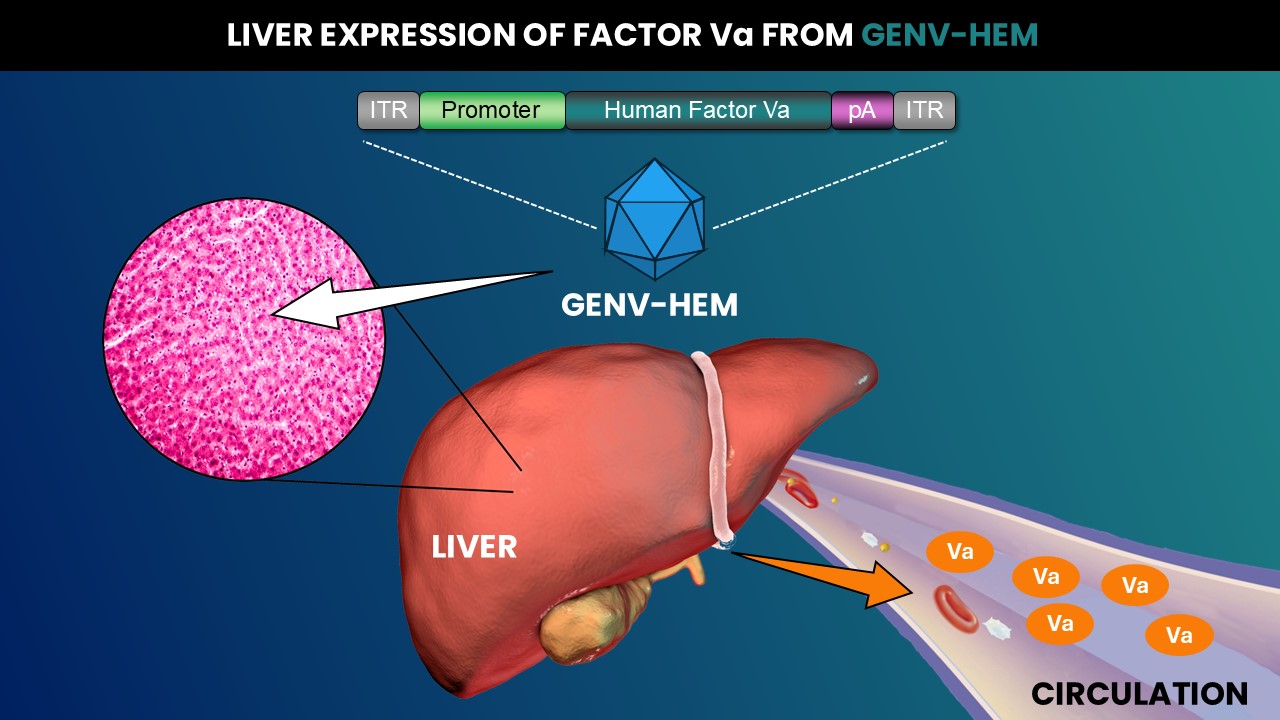
GENV-HEM is an Adeno-associated virus (AAV) serotype 8 vector that contains the DNA sequence for human activated Factor V (Va). The Va sequence is expressed from a liver-specific promoter and contains a polyadenylation (pA) signal, flanked by inverted terminal repeats (ITRs). Administration of GENV-HEM results in hepatocyte transduction in the liver. Existing (approved or in development) AAV-based approaches with Factor VIII direct its non-native expression in transduced cells. In contrast, hepatocytes transduced with GENV-HEM can natively express high levels of endogenous Factor V and GENV-HEM-directed Factor Va, avoiding potential mechanisms linked to decreased expression long-term.
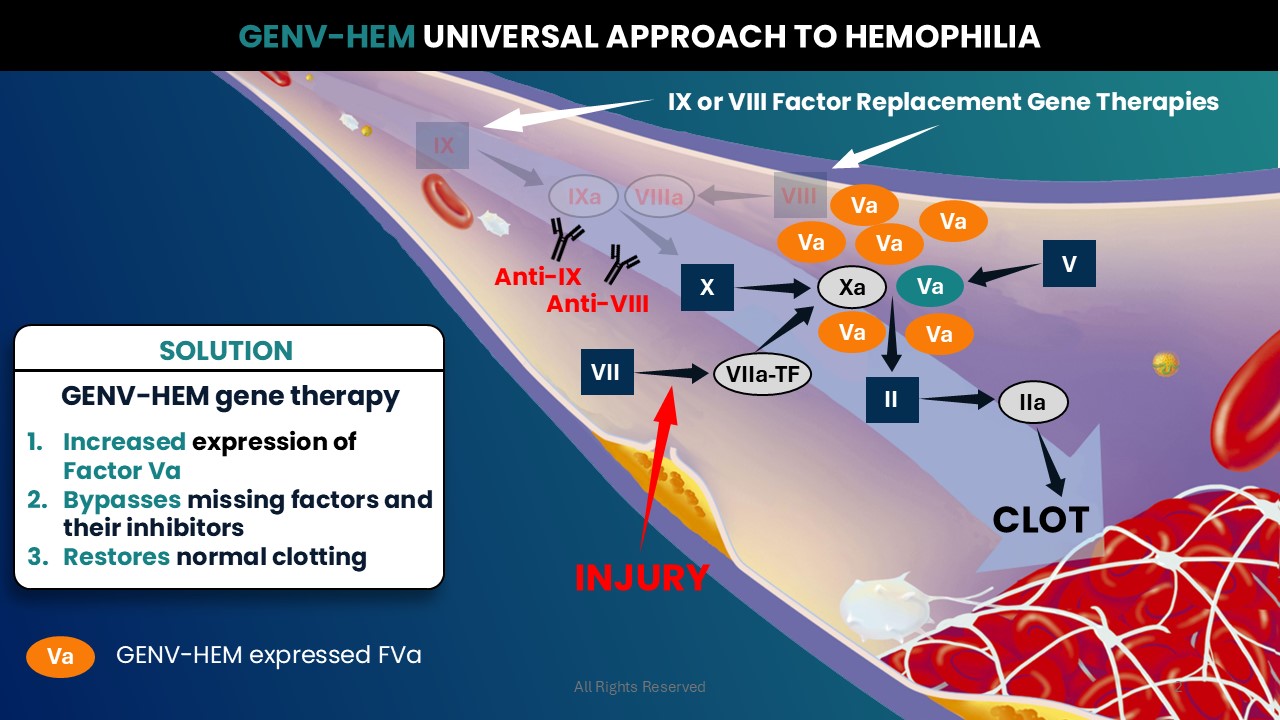
Administration of GENV-HEM results in expression of Factor Va. After an injury, locally generated Va and GENV-HEM expressed Va complex with local Xa to generate IIa and result in clot formation. The combined pool of Va is subjected to the highly regulated endogenous anticoagulation pathway, resulting in GENV-HEM’s normal pre-clinical safety profile. Since GENV-HEM acts downstream of reactions with Factor VIII or IX, it can bypass the missing factors in hemophilia and their inhibitors. This makes GENV-HEM a universal approach for hemophilia.
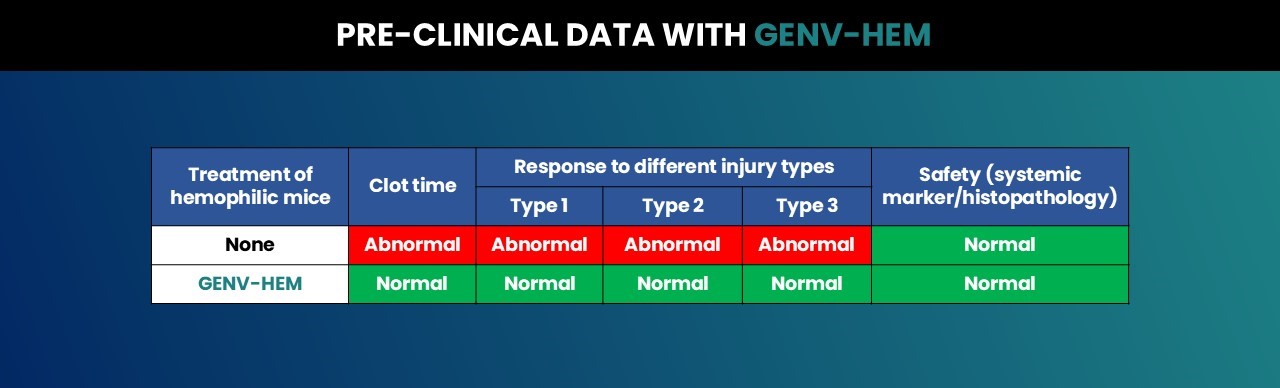
In pre-clinical studies in hemophilic mice (with or without inhibitors), administration of GENV-HEM resulted in normalization of clot time and responses to three widely used injury types. Administration of GENV-HEM resulted in a normal safety profile over a wide range of AAV doses.